H2Po4 Lewis Structure. When we have an h (or h2) in front of a polyatomic molecule (like co 3, so 4, no 2, etc.) we know that it's an acid. Not available fraction sorbed to airborne particulates (phi):
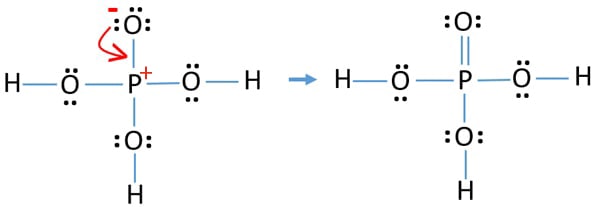
P and o both need 8 valence electrons to be happy (octet rule) which means that you need a total of 40 electrons (5 molecules * 8. When we have an h (or h2 or h3) in front of a polyatomic molecule (like co 3, po 4, no 2, etc.) we know that it's an acid. Dihydrogenphosphate is a monovalent inorganic anion that consists of phosphoric acid in which one of the three oh groups has been deprotonated.
> You Can Find The Procedure Here.
This means that the hydrogen atoms will be attached to the outside of the oxygen molecules. When we have an h (or h2) in front of a polyatomic molecule (like co 3, so 4, no 2, etc.) we know that it's an acid. It is a conjugate acid of a hydrogenphosphate.
This Is Neither Satisfying Octet Rule Nor Putting Negative Charge On Most Electronegative Element.
Answer the following questions based on your lewis structure(s) (a) how many valence electrons are in your lewis drawing? Answer the following questions based on your lewis structure(s) (a) how many valence electrons are in your lewis drawing? One hydrogen atom is joint to phosphorous atom and remaining hydrogen atoms are joint to two oxygen atoms.
This Means That The Hydrogen Atoms Will Be Attached To The Outside Of The Oxygen Molecules.
Is this not the case? Echemi.com offers a wide variety of articles about h2po4 lewis structure, easily find your h2po4 lewis structure information here online. Here's how i would do it.
You Can Draw One That Puts The Negative Charge Onto The The Phosphorus.
When we have an h (or h2) in front of a polyatomic molecule (like co3. Actually, i was drawing resonance structure of $\\ce{h3po4},$ but got confused. H 2 po 4 (each o atom is bonded to the p atom, and each h atom is bonded to an o atom)
The Trial Structure Is You Have 20 Valence Electrons In Your Trial Structure.
(c) how many energy equivalent resonance contributors are there? When we have an h (or h2 or h3) in front of a polyatomic molecule (like co 3, po 4, no 2, etc.) we know that it's an acid. When we see that we have h's in front of a polyatomic ion, we know that the h's are going to be attached to the outside of the oxygens to form oh groups.