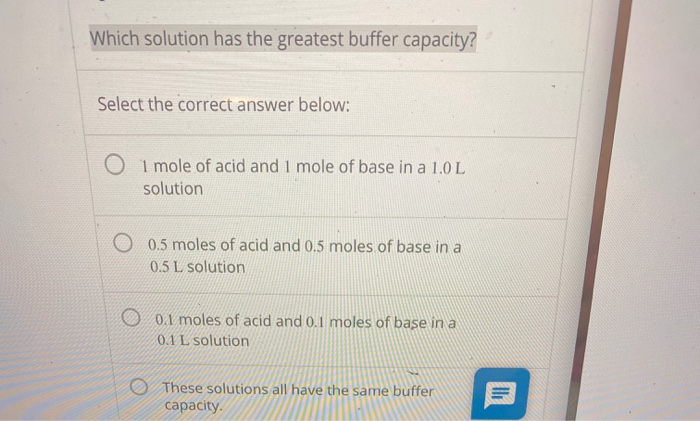
Greatest Buffering Capacity. For example, 0.5 m ch3coona/0.5 m ch3cooh. As one gets more than two units above or below the pk, however, other species become involved and the calculations get more complicated.
So, to give a more clear definition, buffer capacity may be defined as the quantity of a strong acid or strong base that must be added to one liter of a solution to change it by one ph unit. 0.121 m hc2h3o2 and 0.116 m nac2h3o2 1 see answer add answer naomilull2434 is waiting for your help. The optimal buffering range for a buffer is the dissociation constant for the weak acid component of the buffer (pka) plus or minus ph unit.
1.18 M Hf And 0.486 M Nafe.
The one that changes the least has the most buffering capacity. 3 years ago | 06/07/2018 16:45:06 | gknowledge. A) 0.121 m hf and 0.667 m naf b) 0.821 m hf and 0.217 m naf c) 0.821 m hf and 0.909 m naf d) 0.100 m hf and 0.217 m naf e) they are all buffer solutions and would all.
Β = Millimoles /(Δph) Problems On Buffer Solution.
That is the one where base = acid. 0.120m hc 2 h 3 o 2 and 0.115 m nac 2 h 3 o 2. (d) 0.821 m hf and 0.909 m naf has the greatest buffering capacity.
The Ph Of This Buffer = 4.74
0.591 m hf and 0.243 m nafd. A pka between 6 and 8. The greatest buffering capacity at physiological ph would be provided which of the following amino acid?
Where N Is Some Equivalents Of Added Strong Base (Per 1 L Of The Solution).
Which of the following solutions has the greatest buffering capacity? So, to give a more clear definition, buffer capacity may be defined as the quantity of a strong acid or strong base that must be added to one liter of a solution to change it by one ph unit. The greatest buffering capacity at physiological p h would be provided by a protein rich in which of the following amino acids?
The Value Of The Buffer Capacity Is Strongly Related To The Concentrations Of Ingredients Used And Increases With Their Increase.
0.365m hc2h3o2 and 0.497 m nac2h3o2 b. 1) 0.820 m hc2h302 and 0.715 m nac2h302 2) 0.120 m hc2h302 and 0.115 m nac2h302 3) 0.520 m hc2h302 and 0.116 m nac2h302 4) 0.335m hc2h302 and 0.497 m nac2h302 this problem has been solved! 0.521 m hc2h3o2 and 0.217 m nac2h3o2 c.