Molar Mass Of Methanol. 32.04186 · g m o l the exact term of the above molecular weight is “molar mass”, which is based on the atomic mass of each element. What is the molecular shape of ch3oh?
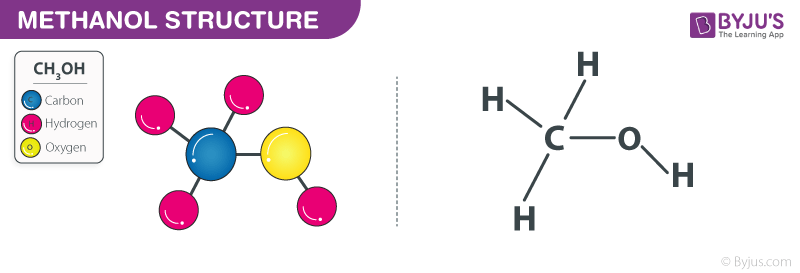
The molar mass of methanol is 32 g/mol. Methanol is a simplest alcohol with a chemical formula ch 3 oh. So molar mass of methanol=12 + (1×3) + 16 + 1 =32g as the chemical formula for methanol or methyl alcohol is ch3oh.
At 20°C (68°F Or 293.15K) At Standard Atmospheric Pressure.
Of 32.042 g/mol), used as a cooking fuel, burns in oxygen to produce carbon dioxide and water. The molar mass of methanol is 32 g/mol. At 20°c (68°f or 293.15k) at standard atmospheric pressure.
About Methanol Methanol Weighs 0.7914 Gram Per Cubic Centimeter Or 791.4 Kilogram Per Cubic Meter, I.e.
About methanol methanol weighs 0.7914 gram per cubic centimeter or 791.4 kilogram per cubic meter, i.e. So molar mass of methanol=12 + (1×3) + 16 + 1 =32g as the chemical formula for methanol or methyl alcohol is ch3oh. Solution for methanol (ch3oh) (molar mass:
Molar Mass / Molecular Weight Of Ch3Oh:
We may be able to balance a chemical equation and determine that one molecule of hydrogen combines with two of oxygen to make water (or the compound of your choice). Add carbon mass with h mass = 12 + 1 x 4 ) g/mol ( as 4 atoms of h ) 12+4 = 16 g/mol. Molar mass of compound methanol (ch3oh)= sum of atomic masses of elements of the compound methanol (ch3oh).
Molar Mass Of Ethanol (C 2 H 5 O H) = 2 (1 2) + 6 (1) + 1 6.
1 grams methanol = 0.031209174498609 mole using the molecular weight calculator and the molar mass of ch3oh. Molar mass of methanol = 12.0107 + (1.00794 × 4) + 15.9994 = 32.04 g/mol (2 decimal places) ∴ moles of methanol burnt = mass of methanol. Molar mass of compound methanol (ch3oh)= sum of atomic masses of elements of the compound methanol (ch3oh).
Molar Mass Of Methanol Molecular Weight Of Ch 3 Oh Formula Formatted Formula Ch 3 Oh Empirical Formula Ch 4 O Molar Mass 32.04 Percents C 37.5% H 12.6% O 49.9% Definitions Used In Online Molar Mass Calculator
The number of moles of methanol present in 160 g of methanol are 32g/mol160g =5mol. The si base unit for amount of substance is the mole. The density of methanol is 791.80 kg/m³ and molar mass is 32.04g/mol.