Lewis No2. Estructura de lewis del no2 (dióxido de nitrógeno) para comenzar a realizar la estructura de lewis del no 2 (dióxido de nitrógeno) vamos a seguir unos sencillos pasos. Because the lone pairs of electrons on the nitrogen atom are mostly responsible for the no2+ molecule geometry planar, we need to calculate out how many there are on the central nitrogen atom of the no2+ lewis structure.
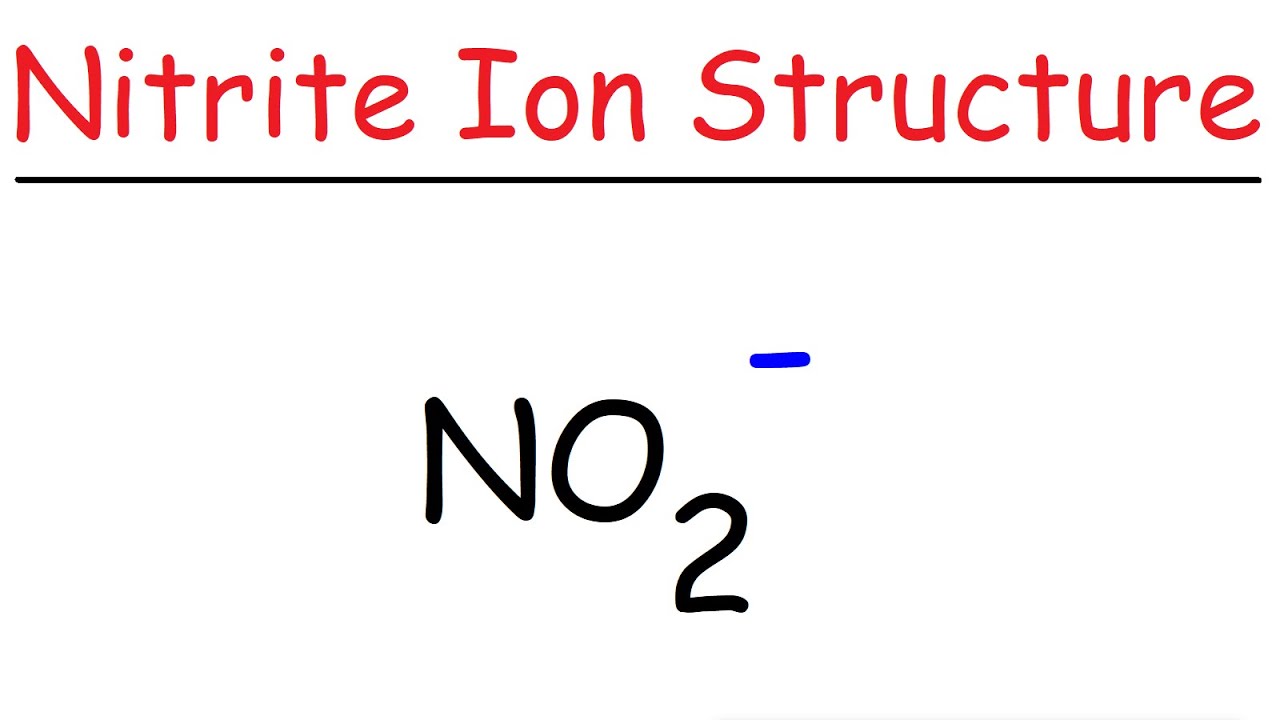
After determining how many valence electrons there are in no2, place them around the central atom to complete the octets. En otros casos se comparten pares de electrones entre los átomos →enlace covalente. Los electrones de la capa de valencia juegan un papel fundamental en el enlace químico.
As With All Other Lewis Dot Structures The Bonds Within The Structure Can Be Replaced By Two Dots.
Similarly, what is the formal charge on the. In the lewis structure for no2 the nitrogen atom is the least electronegative atom and goes at the center of the structure. For the no2 lewis structure, calculate the total number of valence electrons for the no2 molecule.
Use The Formula Below To Find The Lone Pair On The Nitrogen Atom Of The No2+ Molecule.
Sin embargo, la concentración debe ser baja ya que hay sospechas de que favorecen el desarrollo de cáncer. Both of the oxygen's on the ends of no2+ contain two lone pairs of electrons and are chemically neutral. Ejercicios enlace covalente 1 tema 6 1.
Cuenta El Número Total De Electrones (N T) De La Capa De Valencia Para Los Átomos De La Molécula.
Estructura de lewis de no 2 +, ion nitronio. Because of this we'll try to get as close to an octet as we can on the central nitrogen (n) atom. Click to read further detail.
No Electrons Pairs Exist On Nitrogen Atom.
Dibuja las estructuras de lewis para los siguientes compuestos: Please find the lewis dot structure for no2+ (also known as nitronium ion) shown above. Because of this we’ll try to get as close to an octet as we can on the central nitrogen (n) atom.
A Partir De Este Modelo Se Desarrolló La Teoría De Lewis:
Hybridization of no2 (nitrogen dioxide) no 2 involves an sp 2 type of hybridization. The geometry of the no2+ molecule can then be predicted using the valence shell electron pair repulsion theory (vsepr theory), which states that molecules will choose the no2+ geometrical shape in which the electrons have from one. Is no2 a lewis acid or base?
Related Posts
- Lewis Structure Chcl3Lewis Structure Chcl3. The hybridization of chloroform is sp³. In the lewis structure, valence electrons are represented by dots, and 2 bonding elect ...
- So4 Lewis Dot StructureSo4 Lewis Dot Structure. The allene molecule has the following lewis structure: We draw lewis structures to predict:Estructura De Kekule Y Lewis 2020 ...
- Xeo2F2 Lewis StructureXeo2F2 Lewis Structure. The ground state of the xenon has 8 electrons arranged in s2 p6 orbitals. But this is not the case, and a lone pair is presen ...
- Na2O Lewis Dot StructureNa2O Lewis Dot Structure. We identified it from reliable source. Here are a number of highest rated sodium oxide lewis dot structure pictures upon in ...
- The Lewis Structure Of No2The Lewis Structure Of No2. This will mean that it will only have 7 valence electrons. Now, every atom has eight electrons but nitrogen can form only ...
- Lewis Structure Of Ch3Nh2Lewis Structure Of Ch3Nh2. You could not isolated going bearing in mind books accretion or library or borrowing from your associates to right of entr ...
- Ch32Nh Lewis Acid Or BaseCh32Nh Lewis Acid Or Base. It is shipped as a liquefied gas under its vapor pressure. I have 0% idea how to do this.Lewis acids and bases YouTube fro ...

