So4 Lewis Dot Structure. The allene molecule has the following lewis structure: We draw lewis structures to predict:
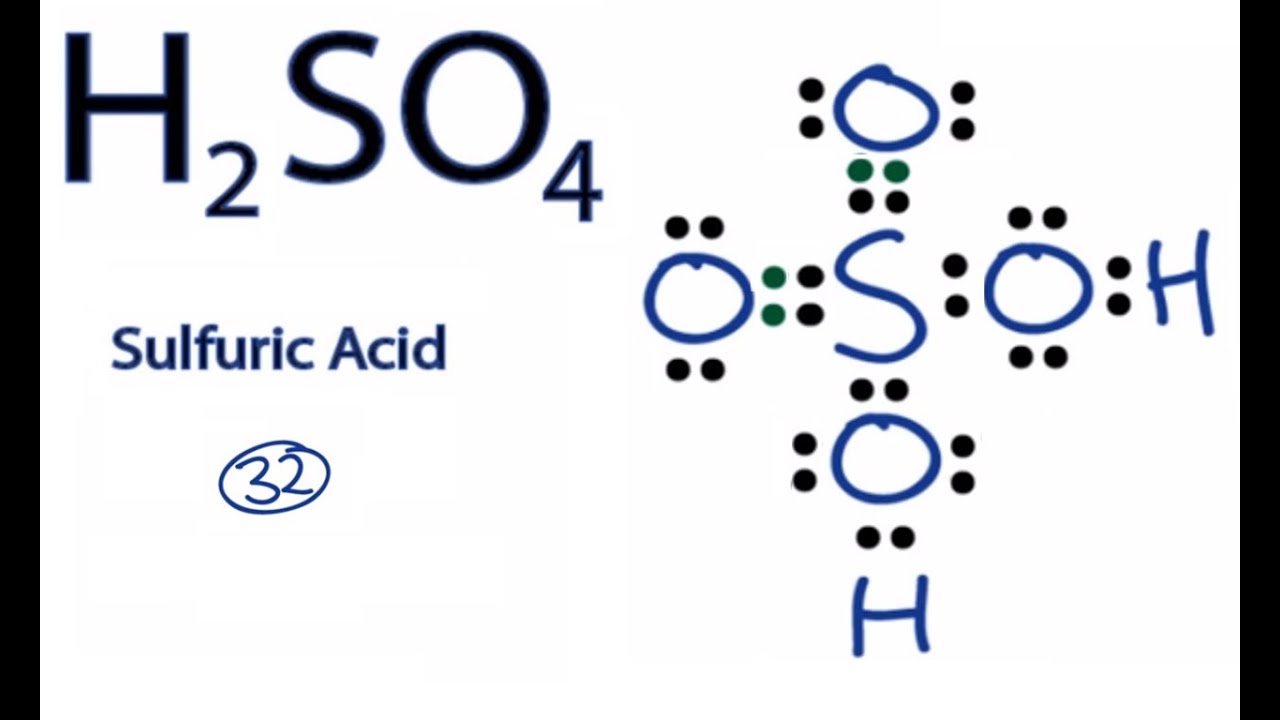
How to draw the lewis dot structure for caso4: Sulfur atom is the center atom and four oxygen atoms are located around sulfur atom. The lewis structure for so is requires you to place more than 8 valence electrons on sulfur (s).
Here, Sulphur Is The Central Atom And It Is Surrounded By Four Oxygen Atoms That Are Located At Equal Distances In The Plane.
First, we will have sulfur in the middle with oxygen surrounding it. What is the lewis symbol for na+? You might think you've got the correct lewis structure for so 4 at first.
What Is The Structure Of So4?
I also go over hybridization, shape and bond angles. Lewis structure of sulfuric acid is drawn step by step in this tutorial. Here sulphur is the central atom and it is surrounded by four oxygen atoms which are located at equal distances in the plane.
There Are No Lone Pairs In The Last Shell Of Sulfur Atom.
The lewis structure for so is requires you to place more than 8 valence electrons on sulfur (s). Sulfur atom is the center atom and four oxygen atoms are located around sulfur atom. Sulfur is in group 6a so it have 6 valence electrons and oxygen has six, so fill this all in around the elements.
The Sulphate Ion Is Mainly Composed Of Sulphur And Oxygen Atoms.
It has less than an octet on at least one atom. Sulfuric acid | h 2 so 4 sulfuric acid is a dibasic strong acid. The electrons, which are negatively charged particles, are present in.
The Allene Molecule Has The Following Lewis Structure:
Put sulphur in the middle with the four oxygens surrounding and just add in the dots. I know that it is missing electrons in order to satisfy the. Its submitted by meting out in the best field.
Related Posts
- Icl4 Lewis Dot StructureIcl4 Lewis Dot Structure. The lewis dot for iodine chloride starts with an i atom in the center. It is a yellow solid which decomposes above −28 °c.B ...
- Dot Structure For NaclDot Structure For Nacl. It occurs in oceans and sea waters. The lewis dot structure for francium up with chlorine's unpaired dot.Lewis Dot Diagr ...
- Clf2 Lewis Dot StructureClf2 Lewis Dot Structure. It tells us how electrons are organized around specific atoms in a molecule. This is the correct structure because the chlo ...
- Ch4 Lewis Structure Polar Or NonpolarCh4 Lewis Structure Polar Or Nonpolar. The electronegativity of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the partial charges t ...
- Xeo2F2 Lewis StructureXeo2F2 Lewis Structure. The ground state of the xenon has 8 electrons arranged in s2 p6 orbitals. But this is not the case, and a lone pair is presen ...
- Cl0 Lewis StructureCl0 Lewis Structure. An atom, molecule, or ion has a formal charge of zero if it has the number of bonds that is typical for that species. The chlori ...
- Predict The Organic Product Of The Following Reaction Include Hydrogen Atoms In Your StructurePredict The Organic Product Of The Following Reaction Include Hydrogen Atoms In Your Structure. Predict the organic product of the following reaction ...


