Ground State Electron Configuration Of Sulfur. Isotope one has a mass of 40.005 amu with a relative abundance of 14.00%. [ne] 3s² 3p⁴ which electron configuration represents an excited state?
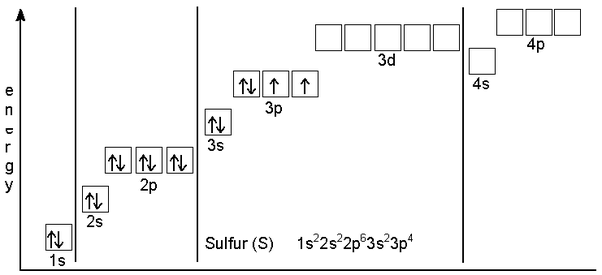
The ground state electronic configuration of s is 3s2 3p4. 32s, 33s, 34s, and 36s. 4d85s1 and the term symbol is 4f9/2.
Sulfur's Electron Configuration Is 1S 2 2S 2 2P 6 3S 2 3P 4.
Sulfur's has an atomic number equal to 16, which means that a neutral sulfur atom has a total of 16 electrons surrounding its nucleus. What is the electron configuration of the excited sulfur atom? What is the ground state electron configuration of sulfur, whose atomic number is 16?
The Valency Of The Element Is Determined By Electron Configuration In The Excited State.
The ground state electronic configuration of s is 3s2 3p4. Express your answer in condensed form, in order of increasing orbital energy. Schematic electronic configuration of sulfur.
Why Is 3D In The.
Hence the s electron configuration is 1s 2. How do you know how many unpaired electrons? The kossel shell structure of sulfur.
For Example, The Names Of The Subshells In A Sulfur Atom Would Be 1S, 2S, 2P, 3S, And 3P (Since Sulfur Has Three Electron Shells).
13 electron configuration s mrsq answers, the aqua group guide to procurement tendering and contract Sulfur also has no charge (neutral element) which means that no electrons are removed or added in the atom. Sulfur atoms have 16 electrons and the shell structure is 2.8.6.
Where Do Absorbed Photons Go?
What happens when an electron goes from excited to ground state? The ground state configuration for the nonmetal sulfur is written as: Since 1s can only hold two electrons the next 2.
Related Posts
- Ground State Electron Configuration Of BoronGround State Electron Configuration Of Boron. How do you find unpaired electrons? The electron configuration, there spa spa 5p3 represents:boron Prop ...
- Give The Electron Configuration For A Neutral Atom Of SeleniumGive The Electron Configuration For A Neutral Atom Of Selenium. Possible compounds with halogens are sef2 and secl2. Correct answer to the question w ...
- Arkansas State Bird FactsArkansas State Bird Facts. It has over 600,000 acres of lakes. The top of four stars in the center represents that arkansas was a member of the confe ...
- Electron Configuration LanthanumElectron Configuration Lanthanum. 58 6s 2 4f 1 5d 1 thorium: Lanthanide ions exhibit emission in the solid state, and in some cases in aqueous soluti ...
- Electron Dot Diagram For Br2Electron Dot Diagram For Br2. Valence electrons and lewis dot structure worksheet answers What is the electron dot structure for br2.What is the Lewi ...
- Chcl3 Electron Dot StructureChcl3 Electron Dot Structure. After determining how many valence electrons there are in chcl3, place them around the central atom to complete the oct ...
- Configuration Of NitrogenConfiguration Of Nitrogen. As such, it gains 3 electrons. If you are still not getting the nitrogen electron configuration of the element nitrogen th ...


