Configuration Of Nitrogen. As such, it gains 3 electrons. If you are still not getting the nitrogen electron configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following;
The first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. 1s 2 2s 2 2p 4:
Some Are Hard To Memorise (Or Predict), So What Is The Electron Configuration Of An Atom Of N?
This video tells about how electronic configuration of nitrogen can be written using s, p, d & f notation, orbital diagram & condensed electronic configurati. The electron configuration of nitrogen is as follows: Electron configuration and oxidation states of nitrogen.
Describe The Structure Of Hydrogen, Carbon, Oxygen And Nitrogen And Relate These To The Structure Of Biological Molecules M1:
In order to be stable, the 3 electrons in the p orbital have to be paired. The nitrogen content and configuration in carbon materials has been changed regularly within a certain range by adjusting the proportion of ionic liquids. 1s 2 2s 2 2p 6:
These 1 4 Electrons Can Be Accommodated In The Various Molecular Orbitals In Order Of Increasing Energy.
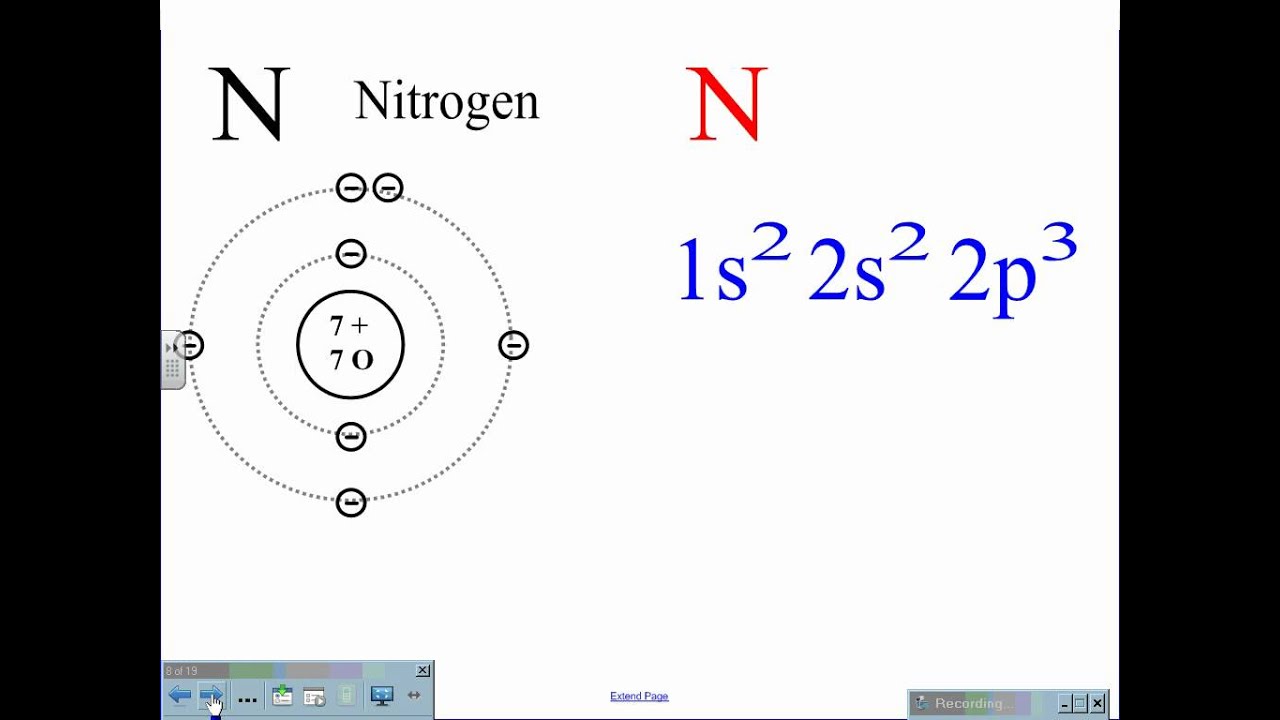
Nitrogen, as a gas, is colorless, odorless, and a generally inert element. The atomic number of the nitrogen is seven, which makes its electronic configuration 1s2 2s2 2p3. Explain the relevance of the electronic configuration of hydrogen, carbon, oxygen and nitrogen hydrogen.
How Would The Electron Configuration Of Nitrogen Change To Make A Stable Configuration?
2b2 is the π* 2py antibonding mo. It has a triple bond and one lone pair on each nitrogen atom. Electron configuration of neon (ne) [he] 2s 2 2p 6:
If You Are Still Not Getting The Nitrogen Electron Configuration Of The Element Nitrogen Then, The Full Electronic Configuration Of Nitrogen Is Written As The Following;
Therefore, the formed noble gas configuration is for neon (ne) and the charge on nitrogen is 3 ( ). As a liquid it is also colorless and odorless, and is similar in appearance to water. 1a1 is the σ2s bonding mo.
Related Posts
- Nitrogen Monoxide Chemical FormulaNitrogen Monoxide Chemical Formula. The chemical formula for nitrous oxide is n 2 o and this formula represents one atom of oxygen and two atoms of n ...
- Electron Configuration For Calcium IonElectron Configuration For Calcium Ion. A calcium 2 + ion has lost its two valence electrons, and now has 18 electrons. Caco3 2hcl forms co2 cacl2 h2 ...
- How Many Covalent Bonds Does Nitrogen Form In Electrically Neutral CompoundsHow Many Covalent Bonds Does Nitrogen Form In Electrically Neutral Compounds. How many covalent bonds does nitrogen form in electrically neutral comp ...
- Ground State Electron Configuration Of BoronGround State Electron Configuration Of Boron. How do you find unpaired electrons? The electron configuration, there spa spa 5p3 represents:boron Prop ...
- Lewis Structure NitrogenLewis Structure Nitrogen. Since it is in group 5 it will have 5 valence electrons. No2 has 5+6+6=17 valence electrons.Nitrogen Facts, Symbol, Discove ...
- Abbreviated Electron Configuration For SulfurAbbreviated Electron Configuration For Sulfur. The abbreviated electron configuration for the sulfur atom is configuraã§ã £ electronics sulfur (s) is ...
- Electron Configuration LanthanumElectron Configuration Lanthanum. 58 6s 2 4f 1 5d 1 thorium: Lanthanide ions exhibit emission in the solid state, and in some cases in aqueous soluti ...


