Write The Condensed Electron Configurations For The Mg Atom. I'll go over how to write the electron configuration both the full electron configuration and condensed/abbreviated noble gas electron configuration. This allows us to determine which orbitals are occupied by electrons in each atom.
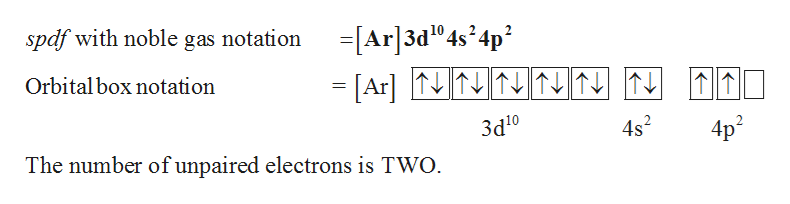
Express your answer in condensed form as a series of orbitals. Write the condensed electron configurations for the ca atom written by henke thipon tuesday, november 23, 2021 add comment edit. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom.
Electronic Configuration Of Mg2+ Tells Us How Many Electrons Are Arranged In Each Shell Of The Mg2+ Atom And Its Shape.
Condensed electron configuration for mg to determine the configuration of electrons for any particular atom, we can ã ¢ â,¬ å build ¢ â,¬ the structures in the order of atomic numbers. Determine in which orbital will be found the last electron of the following elements: For example, [he]2s22p2 should be entered
For Example, [He]2S22P2 Should Be Entered As [He]2S^22P^2.
In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). Write the complete electron configurations and the condensed configurations for mg, s and k. Express your answer in condensed form as a series of orbitals.
See All Problems In The Electron Configuration:
Part b write the condensed electron configurations for the sn atom. It is the 20th element and it has 20 electrons. Gallium element with symbol ga has an atomic number of 31.
The 1S Orbital Can Accommodate Two Electrons.
The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ). Express your answer in condensed form in order of increasing orbital energy as a string without blank space between orbitals. Each of the following electron configurations represents an atom in an excited state.
1S 2 2S 2 2P 6 3S 2 3P 6 4S 2 3D 10 4P 6 5S.
Our tutors have indicated that to solve this problem you will need to apply the the electron configuration: In writing the electron configuration for calcium the first two electrons will go in the 1s orbital. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons).
Related Posts
- Abbreviated Electron Configuration For SulfurAbbreviated Electron Configuration For Sulfur. The abbreviated electron configuration for the sulfur atom is configuraã§ã £ electronics sulfur (s) is ...
- Boron Atom DiagramBoron Atom Diagram. A bohr diagram can be used to visually show the bohr model of a particular atom. Hence, put the boron atom at the central positio ...
- How Many Valence Electrons Are In An Atom Of LithiumHow Many Valence Electrons Are In An Atom Of Lithium. It has a valence of 1. Electrons equal to protons are located in a circular shell outside the n ...
- Electron Configuration LanthanumElectron Configuration Lanthanum. 58 6s 2 4f 1 5d 1 thorium: Lanthanide ions exhibit emission in the solid state, and in some cases in aqueous soluti ...
- Electron Dot Diagram For Br2Electron Dot Diagram For Br2. Valence electrons and lewis dot structure worksheet answers What is the electron dot structure for br2.What is the Lewi ...
- Ground State Electron Configuration Of SulfurGround State Electron Configuration Of Sulfur. Isotope one has a mass of 40.005 amu with a relative abundance of 14.00%. [ne] 3s² 3p⁴ which electron ...
- How Many Valence Electrons Are In An Atom Of ChlorineHow Many Valence Electrons Are In An Atom Of Chlorine. Fuel economy of the 2004 mazda rx 8 miles per gallon, fuel economy of the 2004 mazda rx. Which ...




