Co3 Molecular Shape. But these electrons are concentrated in three places. It has three resonance structures as the double bond between the oxygen atom and carbon can be placed between any of.
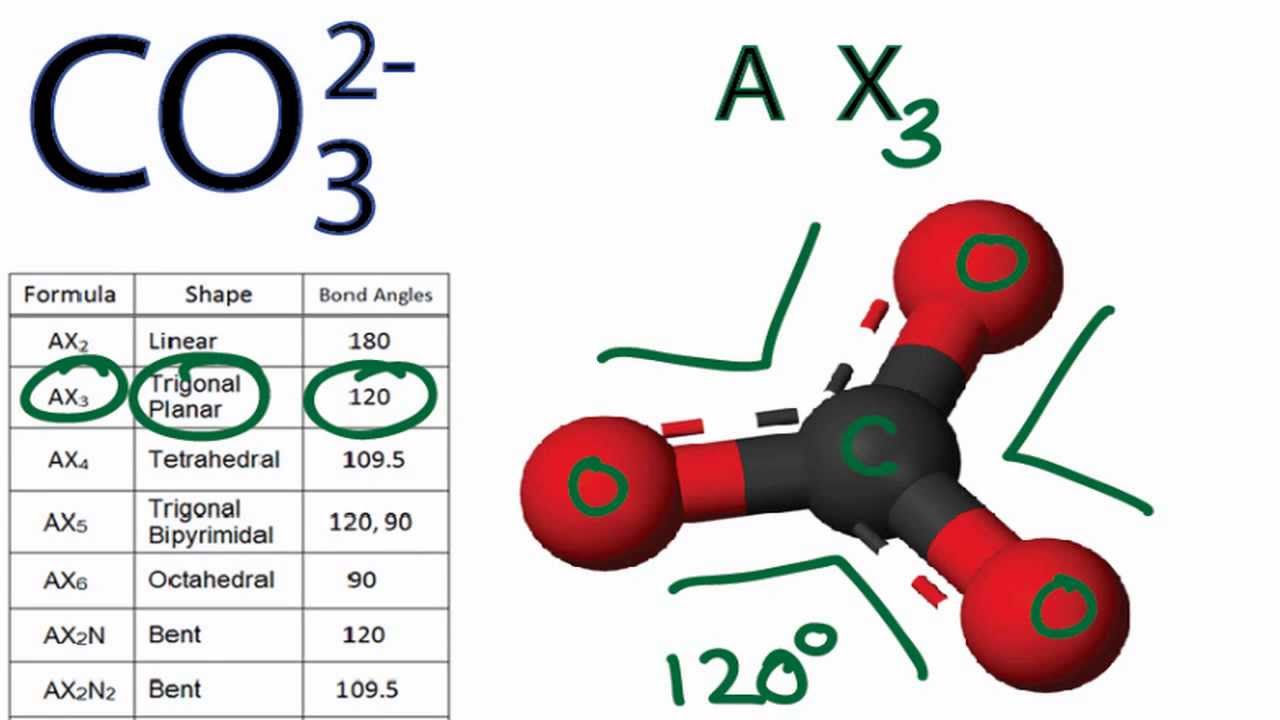
Is co3 positive or negative? En la fórmula química puede utilizar: Planar, trigonal pyramidal, tetrahedral, trigonal planar (120^0),linear.
It Would Have Electron Geometry Trigonal Planar, And A Molecular Geometry Of Bent.
This structure is incompatible with the observed symmetry of the ion which implies that the three bonds are equally long and that the three oxygen atoms are equivalent. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal eg. As a result, there are.
The Shape Of Theorbitals Is Planar Triangular.
Is co3 a trigonal planar? Of a hydrogen atom on methane. The possible isomers of carbon trioxide include ones with molecular symmetry point groups cs, d3h, and c2v.
The C2V State, Consisting Of A Dioxirane Has Been Shown To Be The Ground State Of The Molecule.
It has three resonance structures as the double bond between the oxygen atom and carbon can be placed between any of. What is the geometry of co3? It shows tetrahedral geometry for the electron pairs geometry and trigonal pyramid the molecular geometry.
The Hybridization Of Bromine Must Be Sp^3.
Planar, trigonal pyramidal, tetrahedral, trigonal planar (120^0),linear. Calculando la masa molar (peso molecular) para calcular la masa molar de un compuesto químico introduzca su formula y haga click en 'compute'. Carbon trioxide should not be confused with the stable carbonate ion.
The Carbonate Ion Is The Simplest Oxocarbon Anionit Consists Of One Carbon Atom Surrounded By Three Oxygen Atoms In A Trigonal Planar Arrangement With D 3H Molecular Symmetryit Has A Molecular Mass Of 6001 Gmol And Carries A Total Formal Charge Of 2.
The average of a double bond and 2 single bonds. Ch3+ has a triangular geometry being sp2 hybridised, whereas all the others have tetrahedral geometry being sp3 hybridised, thus ch3+ has a. Also know, what is the molecular geometry of co3 2?
Related Posts
- Sodium Nitrate Molecular MassSodium Nitrate Molecular Mass. Commonly referred to as chile saltpeter this compound consists of a sodium cation na and a nitrate anion no 3. It solv ...
- Molecular Shape Pcl3Molecular Shape Pcl3. The molecular shape of the phosphorous trichloride (pcl3) is trigonal pyramidal, and electronic geometry is tetrahedral, based ...
- Molecular Weight Of C2H4Molecular Weight Of C2H4. It is either ethylene c2h4, carbon monoxide co or nitrogen n2. ¿es esta web útil para ti?C2h4 Molar Mass from virallistclub ...
- Ax4E2 Molecular GeometryAx4E2 Molecular Geometry. Two orbitals are arranged along the vertical axis at 90ofrom the equatorial orbitals. Also asked, what shape is ax4e2?PPT C ...
- 100 Sided Shape Name100 Sided Shape Name. The relationship between the number of faces, edges and vertices of a 3d shape is given by: They are made of straight lines, an ...
- Molecular Geometry For Xef4Molecular Geometry For Xef4. From the overall molecular geometry and the presence and arrangement of polar bonds (if any), determine if a molecule is ...
- Molecular Geometry Cocl2Molecular Geometry Cocl2. All chemistry practice problems molecular geometry practice problems. Nocl consists of one nitrogen atom, one oxygen atom, ...

