Bcl3 Lewis Acid Or Base. Clear all bcl3 lewis acid bf3 lewis base ph3 can act as either a lewis acid or lewis base h20 neither a lewis acid or lewis base cc14. Ce bcl3 can be seen as a hard acid and would thus preferably interact with hard bases.
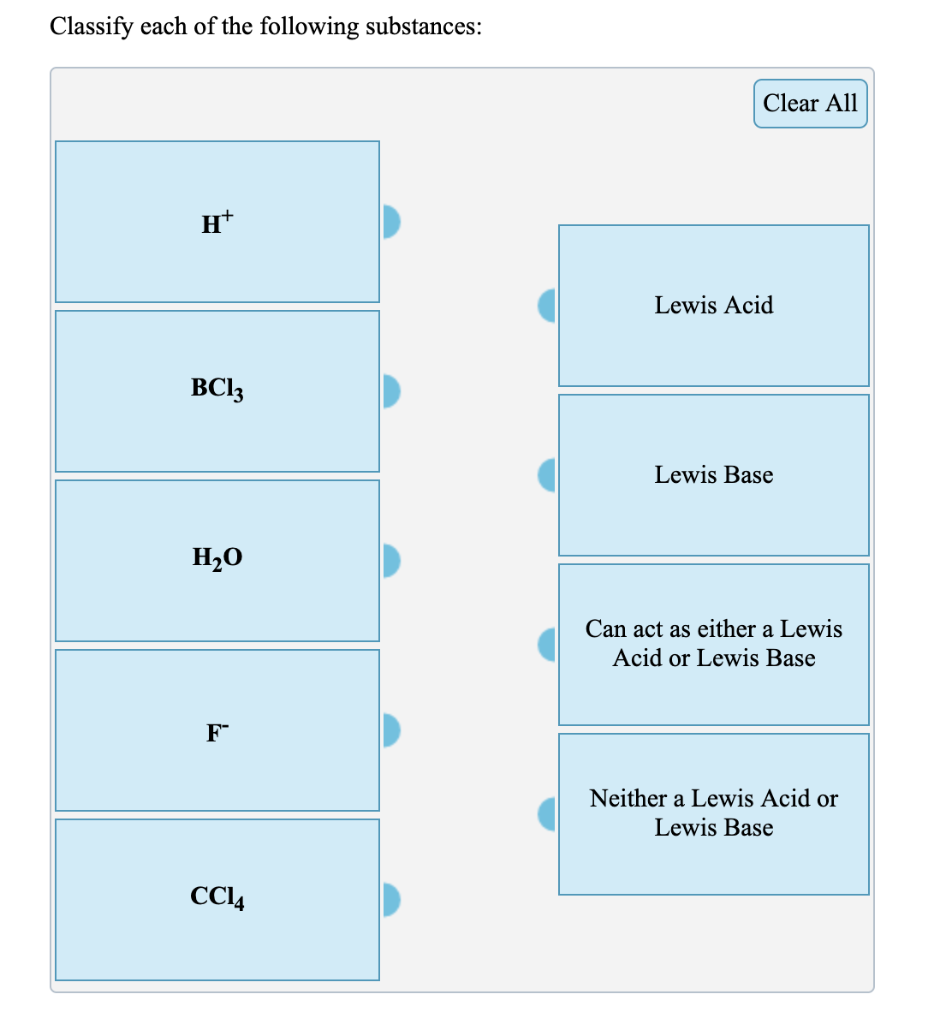
In this reaction, ammonia donates electrons and is, therefore, a lewis base whereas bcl3 accepts electrons and acts as a lewis acid. Why is bcl3 a lewis acid? Clear all bcl3 lewis acid bf3 lewis base ph3 can act as either a lewis acid or lewis base h20 neither a lewis acid or lewis base cc14.
In This Reaction, Ammonia Donates Electrons And Is, Therefore, A Lewis Base Whereas Bcl3 Accepts Electrons And Acts As A Lewis Acid.
Is bcl3 a lewis base? Join / login >> class 11 >> chemistry >> equilibrium >> acids, bases and salts >> in the reaction, bcl3 + ph3 cl3b ph3 , question. Ce bcl3 can be seen as a hard acid and would thus preferably interact with hard bases.
Bcl3 Lewis Acid Or Base.
As a result, a lewis base can transfer two electrons to a lewis acid, resulting in a product with a coordinate covalent bond. \[{{h}_{2}}s\] is a lewis acid that may also be used as a. According to the lewis definition.
Carbon Accepts A Pair Of Electrons, So Co 2 Is The Lewis Acid.
Lewis acids accept an electron pair. $\ce {bcl3}$ can be seen as a hard acid and would thus preferably interact with hard bases. A lewis adduct is another name for this substance.
When Bonding With A Base The Acid Uses Its Lowest Unoccupied Molecular Orbital Or Lumo (Figure 2).
In the lewis approach, a [lewis] acid is an electron pair acceptor (lumo) and a [lewis] base is an electron pair donor (homo). In this reaction, each chloride ion donates one lone pair to becl 2, which has only four electrons around be. The acid is a proton donor, and the base is a proton acceptor.
There Are 3 Main Reasons For This:
The chloride ion contains four lone pairs. We have reinvestigated the relative acid strengths of bf 3 and bcl 3 toward lewis bases by calculating geometries and atomic charges for the following adducts. Also to know is, is hydronium a lewis acid?
Related Posts
- Icl4 Lewis Dot StructureIcl4 Lewis Dot Structure. The lewis dot for iodine chloride starts with an i atom in the center. It is a yellow solid which decomposes above −28 °c.B ...
- Lewis Structure Of Ch3Nh2Lewis Structure Of Ch3Nh2. You could not isolated going bearing in mind books accretion or library or borrowing from your associates to right of entr ...
- Hemlock Base Shoe MouldingHemlock Base Shoe Moulding. Base shoe and base cap are used to conceal uneven floor and wall junctions. Interior moulding applied about one third up ...
- Which Of The Following Is The Best Lewis Symbol For OxygenWhich Of The Following Is The Best Lewis Symbol For Oxygen. Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is i ...
- Lewis Structure For Hso4Lewis Structure For Hso4. The formal charge on : What is the electron dot formula of c2h4?HClO4의 루이스 구조. Lewis structure of HClO4 from ywpop.tistory. ...
- Lewis Structure N2O2Lewis Structure N2O2. For the no2 lewis structure, calculate the total number of valence electrons for the no2 molecule. How to draw the lewis struct ...
- Lewis Structure For HocnLewis Structure For Hocn. 6 years ago i have a recommen. Make sure you have chosen the best structure.Solved Match Each Of The Possible Lewis Structu ...
