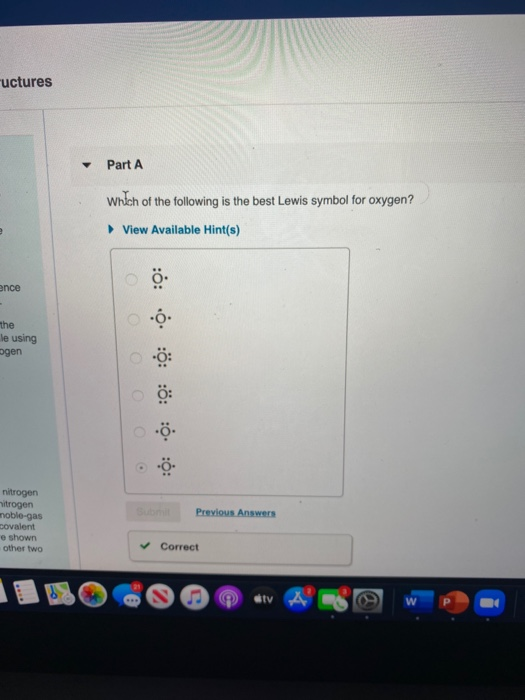
Which Of The Following Is The Best Lewis Symbol For Oxygen. Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a noble gas (which one?). Uctures part a which of the following is the best lewis symbol for oxygen?
Since it is bonded to only one carbon atom, it must form a double bond. Of course the elemental form is bimolecular. Part a which of the following is the best lewis symbol for oxygen a b c d e f from chm 151 at forsyth technical community college
The Reactivity Of The Compound Is Also Consistent With An Electron Deficient Boron.
It has a lone pair of electrons on the boron atom. Dispersion forces which are present in all molecules. Medium answer correct option is c lewis symbol is represented by the atomic symbol with a number of dots around it which is equal to the number of valence electrons.
A Lewis Diagram Depicts A Mmolecule Using An Element Symbol To Represent The Nucleus And Core Electrons Of Each Atom.
14.write the singly bonded lewis dot structure for bf3. View available hint (s) ence the le using ogen o ooooo có: Which of the following is the best lewis dot structure for chlorine?
During Chemical Bonding It Is The Valence Electrons Which Move Amongst Different Atoms.
Oxygen is in group 16/via, so it has six valence electrons. It obeys the octet rule on all atoms. The following procedure can be followed to derive lewis diagrams for most molecules.
A Lewis Symbol Consists Of An Elemental Symbol Surrounded By One Dot For Each Of Its Valence Electrons:
Figure 1 shows the lewis symbols for the elements of the third period of the periodic table. View available hint (s) o b submit 山。. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions.
Sulfur Is In Group 16 (Sometimes Called Group Vi Or 6A).
And thus the neutral atom has 7 valence electrons. How many electrons should be shown in the lewis symbol for hydrogen? View available hint (s) o.
Related Posts
- Hypobromous Acid Lewis StructureHypobromous Acid Lewis Structure. Answers, hypobromous acid wikipedia, chapter 15 acids and bases acids and bases, category hypobromous acid wikimedi ...
- Which Transformation Will Be Equivalent To Rotating A Figure 270° CounterclockwiseWhich Transformation Will Be Equivalent To Rotating A Figure 270° Counterclockwise. Note that a geometry rotation does not result in a change or size ...
- H2Po4 Lewis StructureH2Po4 Lewis Structure. When we have an h (or h2) in front of a polyatomic molecule (like co 3, so 4, no 2, etc.) we know that it's an acid. Not ...
- Contemporary Psychology Is Best Defined As The Scientific Study OfContemporary Psychology Is Best Defined As The Scientific Study Of. Psychology is the science of. Since about 1920, most university psychologists hav ...
- Which Of The Following Are Characteristics Of Impressionist MusicWhich Of The Following Are Characteristics Of Impressionist Music. Debussy was highly influenced by. Which of the following is not a characteristic o ...
- Dauntless Best AxeDauntless Best Axe. To make the most of an axe a slayer must learn to make the most of each opening, committing time to empowering attacks when they ...
- Clf2 Lewis Dot StructureClf2 Lewis Dot Structure. It tells us how electrons are organized around specific atoms in a molecule. This is the correct structure because the chlo ...
